Humoral Response Assessment for SARS-CoV-2 and Variants
Evaluation of humoral response to spike protein, RBD, and nucleocapsid is critical in
SARS-CoV-2 vaccine and therapy research. Assess IgG and IgA response and viral neutralization quickly and accurately with assays used by many of the top SARS-CoV-2 vaccine developers.

Pseudotyped Neutralization Assay
ELISA/MSD
- Assess IgG responses in animal models (mouse, ferret, hamster, NHP) or humans
- Optimized for development, qualified and validated for clinical studies
- Automated liquid handlers
- WHO/NIBSC calibration
- IgA assays forthcoming
- MSD 4-spot
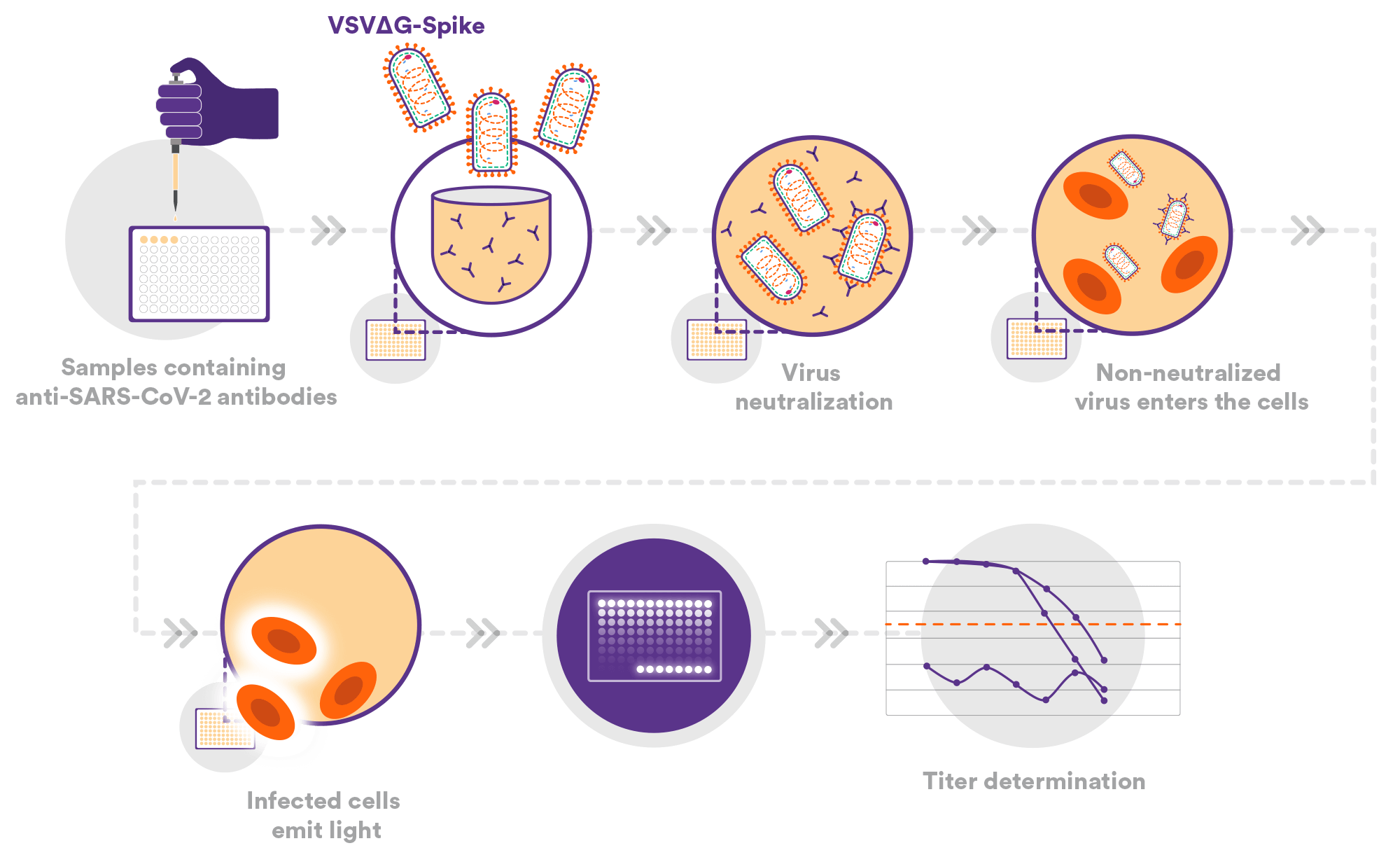
Viral Neutralization Assays (VNA) / Pseudo Neutralization Assay (PNA)
We have established a PRNT assay using a wild-type SARS-CoV-2 strain. Running routinely at Public Health England BSL-3, it is being used to evaluate several drug and vaccine candidates.
We have also developed a pseudo-particle neutralization assay (PNA) using VSVΔG spike. This assay is calibrated to the WHO/NIBSC reference standard and has been evaluated as part of the Duke University SARS-CoV-2 Neutralization Assay Concordance Survey (SNACS). The pseudo-particles associated with the SARS-CoV-2 virus and its variants are generated at a Nexelis facility.

