Translational Science
Custom Assay Development to Advance Molecules
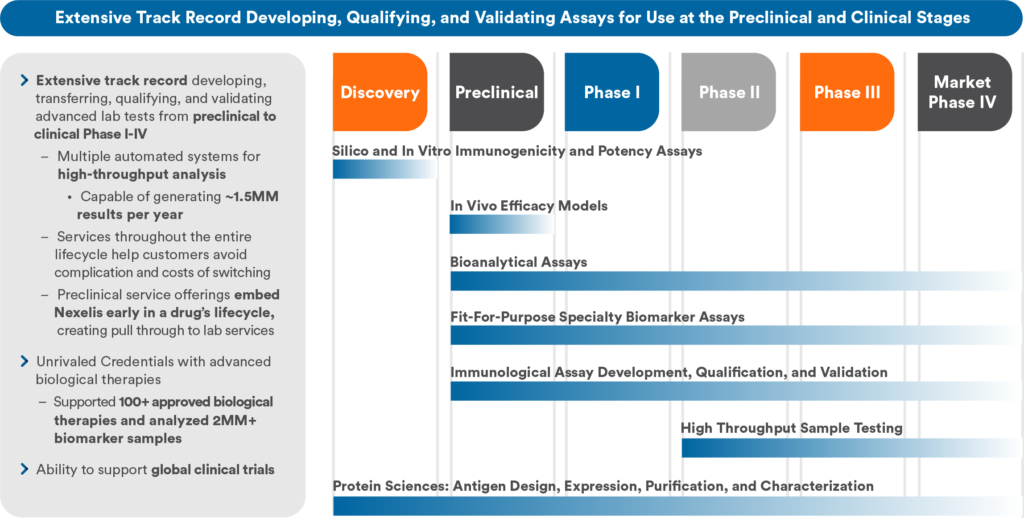
You need reliable approaches to support what’s next in your development programs whether they are complex small molecules, advanced biologics, or vaccines. At Nexelis, a Q² Solutions Company, we break down barriers to translational research through innovation and collaboration, helping you deliver better treatments to more patients, sooner. With an extensive track record developing, qualifying, validating, and transferring tests, our dedicated scientists support programs, from early development to clinical Phase I-IV.

Prepare for Clinical Scale — and for the Unexpected
Along the path from preclinical to clinical trials, many transitions take place. Stable and robust methods yield reliable results. Whether you must evaluate efficacy of an emerging virus or cancer cells’ facile adaptability, your assays must support your candidate, discovery through post-market registration. Throughout Nexelis, at every site, in every business line, and at every phase, we deliver custom assays.
Scalable Assay Platforms From Pilot to High Throughput
Our Global Footprint Means You Have Access to Nexelis’ Services, Wherever You Are
Wherever you do business, you need to be confident of the same level of scientific expertise, transparency, and responsiveness. Our global team of scientists, technology platforms, and advanced network of laboratories offer this to help further the future of patient therapies, including biologics, vaccines, antiviral/antibacterial drugs, immunotherapy, and gene and cell therapy products.
Consistent Platforms In-House and Across Sites Ensure the Best Solutions
At Nexelis, our Protein Science team’s high-throughput production services provide customized critical reagents, such as proteins, for our clients and our assay development teams. We have experience with production and labeling of a wide range of molecules including large, complex proteins on a variety of expression platforms.
- Multiple proprietary or custom in vivo models allow preclinical assessment of immunogenicity and/or efficacy of candidates based on a variety of criteria to support antigen/antibody production. These include viral and bacterial infectious disease models, oncology models, and auto-immune disease models.
- State-of-the-art, multiplex instrumentation ensures efficiency, quality, and the best fit for your needs.

Mesoscale Discovery (MSD)
For biomarker, immunogenicity, PK, and cell-based assays, MSD provides higher sensitivity than conventional colorimetric methods. It also enables multiplexing and is compatible with complex matrices.
Luminex
This robust form of immunoassay enables precise detection of up to 100 analytes simultaneously. This allows quantification of numerous different biomarkers in one sample, revealing information about biological and disease processes. It is especially useful for bioanalysis, such as PK, biomarker, and immunogenicity studies.
ELISpot
An enzyme-linked immune absorbent spot (ELISpot) assay quantitatively measures cellular functions in immune cells. It can be used to assess adaptive and innate immune responses, e.g., to vaccines, via measurement of antigen-specific T-cells. This highly adaptable method is very sensitive to a wide range of responses.
Learn more about infectious disease, vaccine, and biomarker applications.
With 30+ years of experience, our end-to-end bioanalytical and biomarker assay services support your preclinical through Phase IV development programs with the ability to support studies under CAP/CLIA, GCP, or GLP. Our scientific team partner and act as an extension of your team to provide you with the most agile and robust solution to advance your program.
