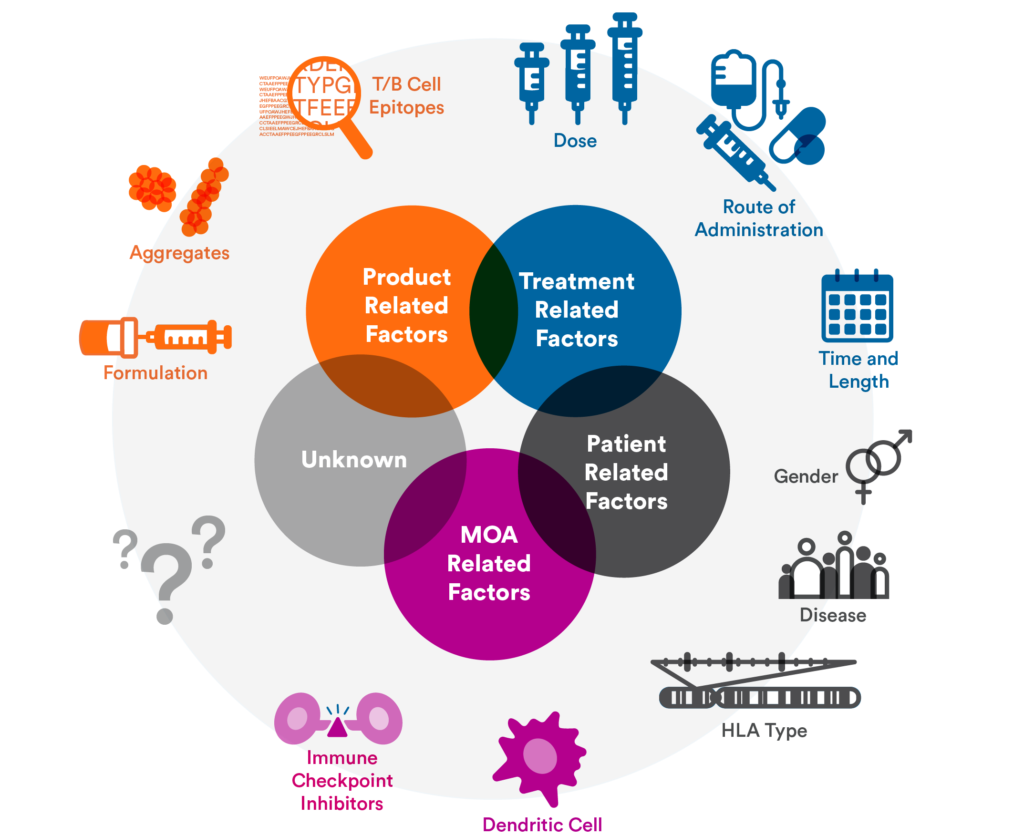
Immunogenicity Assessment: In Vitro Assays and Computational Analysis
ImmunXperts, a Q2 Solutions company, provides trusted immunogenicity risk assessment services, including in vitro methods with T-cell proliferation as surrogate to measure the immunogenic potential for drug candidates, in silico methods for rapid, low-cost assessment of large number of lead candidates, MHC-associated peptide proteomics (MAPPS), and anti-drug antibody (ADA) assays. ImmunXperts also performs functional screening assays for immuno-oncology candidates and risk assessment and mitigation of drug candidates’ immunogenicity profiles. ImmunXperts is a trusted leader in supporting immuno-oncology drug discovery.
In Silico
Computer-based tools for profiling and lead optimization allow fast, sequence-based, early-stage modeling to assess immunogenicity in large numbers of lead candidates. We use state-of-the-art, well-documented, and benchmarked immunological bioinformatics tools for algorithmic evaluation. These advanced tools, which are constantly being optimized by Prof. Morton Nielsen’s lab at the Technical University of Denmark, are ideal for:
- T-cell epitope prediction
- Immunogenicity risk assessment
- Deimmunization
- Customization
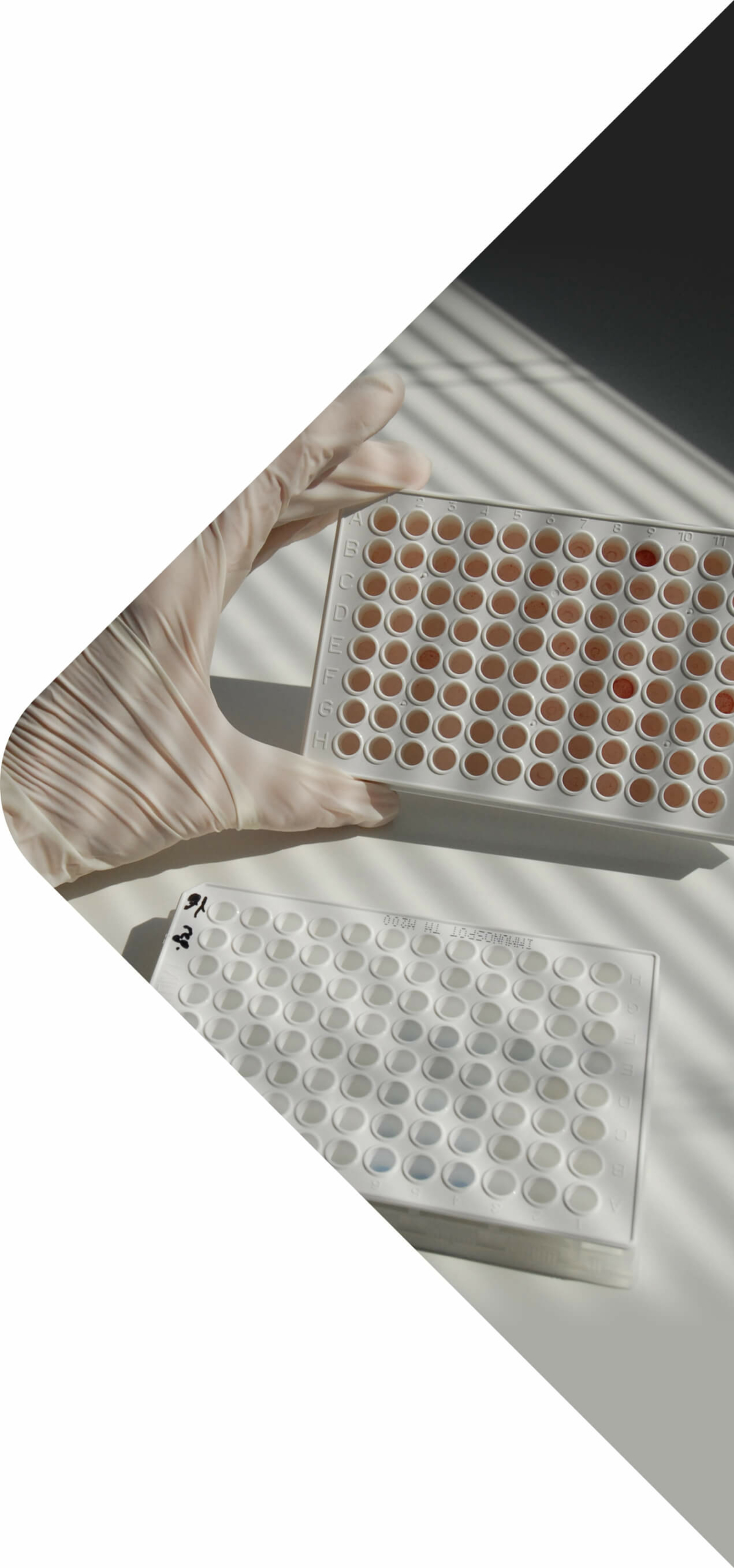
In Vitro
The in vitro immunogenicity services in our laboratory provide mechanistical and functional insights in immune-mediated adverse effects. Our assays include:
- Peripheral Blood Mononuclear Cell (PBMC) assay
- Dendritic Cell (DC-T) assay
- Human Lymphocyte Activation assay
- Whole blood and PBMC cytokine release assay
- MHC-associated Peptide Proteomics (MAPPS) assay
- Dendritic Cell (DC) activation assay
- Reporter cell line assays
Early Immunogenicity Assessment Tools